Rat PDGFRB / PDGFR-1 Protein (Fc Tag)
PDGFRB, Pdgfr, Pdgfr1
- 100ug (NPP3096) Please inquiry
| Catalog Number | P80347-R02H |
|---|---|
| Organism Species | Rat |
| Host | Human Cells |
| Synonyms | PDGFRB, Pdgfr, Pdgfr1 |
| Molecular Weight | The recombinant rat PDGFRB/Fc is a disulfide-linked homodimer. The reduced monomer comprises 740 amino acids and has a predicted molecular mass of 83.2 kDa. The apparent molecular mass of the protein is approximately 117 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Leu 32 |
| SDS-PAGE | 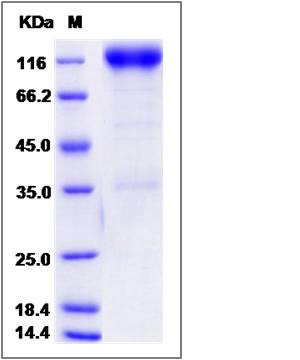 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the rat PDGFRB (Q05030) (Met1-Lys530) was expressed with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | |
| Research Area | Cancer |Invasion microenvironment |Angiogenesis |Growth Factor & Receptor |Platelet-Derived Growth Factor (PDGF) & Receptor |PDGF Receptor | |
| Formulation | Lyophilized from sterile PBS, pH 7.4. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | The cluster of differentiation (CD) system is commonly used as cell markers in immunophynotyping. Different kinds of cells in the immune system can be identified through the surface CD molecules which associating with the immune function of the cell. There are more than 320 CD unique clusters and subclusters have been identified. Some of the CD molecules serve as receptors or ligands important to the cell through initiating a signal cascade which then alter the behavior of the cell. Some CD proteins do not take part in cell signal process but have other functions such as cell adhesion. CD140b, also known as PDGFRB, is a member of the CD system. CD140b is a cell surface tyrosine kinase receptor essencial for development interacting with the platelet-derived growth factors (PDGFs) which serves as mitogens for mesenchymal cells. CD140b can bind with platelet-derived growth factor (PDGF)-B, that are secreted by tumors and phosphorylation of PDGFR-β was correlated with depth of cancer invasion at statistically significant level. |
| Reference |
