Rhesus AMY2A / Alpha-amylase Protein (Fc Tag)
AMY2A,AMY2B
- 100ug (NPP2893) Please inquiry
| Catalog Number | P90012-C02H |
|---|---|
| Organism Species | Rhesus |
| Host | Human Cells |
| Synonyms | AMY2A,AMY2B |
| Molecular Weight | The recombinant rhesus AMY2A comprises 737 amino acids and has a calculated molecular mass of 82.8 KDa. |
| predicted N | Gln 16 |
| SDS-PAGE | 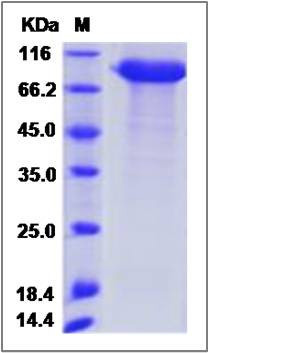 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the rhesus AMY2A (H9EX43) (Met1-Leu511) was expressed with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | |
| Research Area | |
| Formulation | Lyophilized from sterile PBS, pH 7.4. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Alpha-amylase is the major form of amylase found in humans and other mammals. Amylases are secreted proteins that hydrolyze 1,4-alpha-glucoside bonds in oligosaccharides and polysaccharides, and thus catalyze the first step in digestion of dietary starch and glycogen. Alpha-amylase hydrolyses alpha bonds of large, alpha-linked polysaccharides, such as starch and glycogen, yielding glucose and maltose. Amylases is widely expressed and is most prominent in pancreatic juice and saliva, each of which has its own isoform of human α-amylase. They behave differently on isoelectric focusing, and can also be separated in testing by using specific monoclonal antibodies. |
| Reference |
