Rhesus HER3 / ErbB3 Protein (Fc Tag)
ErbB3
- 100ug (NPP2901) Please inquiry
| Catalog Number | P90043-K02H |
|---|---|
| Organism Species | Rhesus |
| Host | Human Cells |
| Synonyms | ErbB3 |
| Molecular Weight | The recombinant Rhesus ErbB3/Fc is a disulfide-linked homodimeric protein. The reduced monomer consists of 865 amino acids and predicts a molecular mass of 95.7 kDa. As a result of glycosylation, rhesus ErbB3/Fc monomer migrates as an approximately 130-140 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Ser 20 |
| SDS-PAGE | 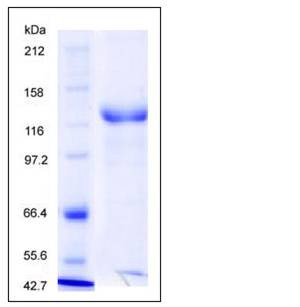 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the Rhesus (Macaca mulatta) ErbB3 (XP_001113953.2) extracellular domain (Met 1-Thr 643) was fused with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | 1. Measured by its ability to human NRG1 (aa 20-241) (P13499-H02H) in functional Elisa. 2. Measured by its ability to human NRG1 (aa 177-241) (P13499-H01H) in functional Elisa. 3. Measured by its ability to human NRG1 (aa 20-241)-His (P13499-H08H) in functional Elisa. |
| Research Area | Cardiovascular |Angiogenesis |Growth Factor & Receptor |Epidermal Growth Factor (EGF) & Receptor |EGF Receptor |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | ErbB3, also known as Her3(human epidermal growth factor receptor3), is a member of the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases. This membrane-bound glycoprotein has a neuregulin binding domain but has not an active kinase domain., and therefore can not mediate the intracellular signal transduction through protein phosphorylation. However, its heterodimer with ErbB2 or other EGFR members responsible for tyrosine phosphorylation forms a receptor complex with high affinity, and initiates the related pathway which lead to cell proliferation or differentiation. ErbB3 has been shown to implicated in numerous cancers, including prostate, bladder, and breast tumors. This protein has different isoforms derived from alternative splicing variants, and among which, the secreted isoform lacking the intermembrane region modulates the activity of membrane-bound form. |
| Reference |
